Abstract
The FDA has issued a warning regarding dosing errors with compounded semaglutide injectable products, stressing significant risks for patients managing type 2 diabetes and using semaglutide for weight loss. Unlike FDA-approved versions, compounded semaglutide varies in concentration and dosing instructions, leading to potential overdoses and severe adverse effects. Clear communication and proper dosing tools are essential to ensure patient safety.
Key Points
- FDA Warning: Alert on dosing errors with compounded semaglutide, noting overdoses and severe side effects.
- FDA-Approved vs. Compounded: Compounded versions vary in concentration and instructions, unlike standardized FDA-approved products.
- Common Errors: Misunderstandings in measurement units and inappropriate syringe use lead to significant overdoses.
- Case Examples: Patients and providers often miscalculate doses, resulting in severe adverse effects.
- Recommendations: Emphasize clear communication, proper dosing tools, and double-checking calculations.
- Summary: Use FDA-approved products and consult healthcare professionals to avoid risks. Report adverse effects to FDA’s MedWatch.
Introduction
The FDA recently issued a warning concerning dosing errors associated with compounded semaglutide injectable products. This alert is particularly relevant for patients managing type 2 diabetes and those using semaglutide for weight loss, as these compounded versions pose significant risks that differ from FDA-approved products.
Background on Semaglutide
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist, prescribed to help manage blood sugar levels in type 2 diabetes (T2D) and for weight loss (WL). The FDA-approved versions of semaglutide include:
- OZEMPIC: Multi-dose pre-filled pens for weekly dosing (T2D).
- RYBELSUS: Oral tablets for daily dosing (T2D).
- WEGOVY: Single-dose pre-filled pens for weekly dosing (WL).
FDA-approved products have clear, standardized doses, ensuring safety and efficacy. Compounded versions vary in concentration and dosing instructions, causing confusion and adding new risks.
Risks of Compounded Semaglutide
The FDA's alert demonstrates several critical issues with compounded semaglutide products:
- Dosing Errors: Reports indicate that patients often miscalculate doses, leading to significant overdoses. This is primarily due to confusion over measurement units (milliliters, milligrams, units) and the use of inappropriate syringes.
- Adverse Events: Overdoses have led to severe side effects, including nausea, vomiting, abdominal pain, dehydration, and in some cases, acute pancreatitis and gallstones. Some patients required hospitalization due to these adverse reactions.
- Inconsistent Instructions: The instructions for compounded semaglutide often vary and may not clearly communicate how to measure and administer the correct dose, leading to further confusion and dosing errors.
- Quality and Safety Concerns: Unlike FDA-approved drugs, compounded medications do not undergo rigorous premarket review. This lack of oversight means that compounded semaglutide can vary in quality and concentration, posing additional risks to patients.
Case Examples
Several reports exemplify the dangers of dosing errors with compounded semaglutide:
- A patient using an insulin syringe administered 50 units instead of the prescribed 5 units, leading to severe vomiting.
- Health care providers have miscalculated doses, sometimes prescribing ten times the intended amount, resulting in multiple adverse events.
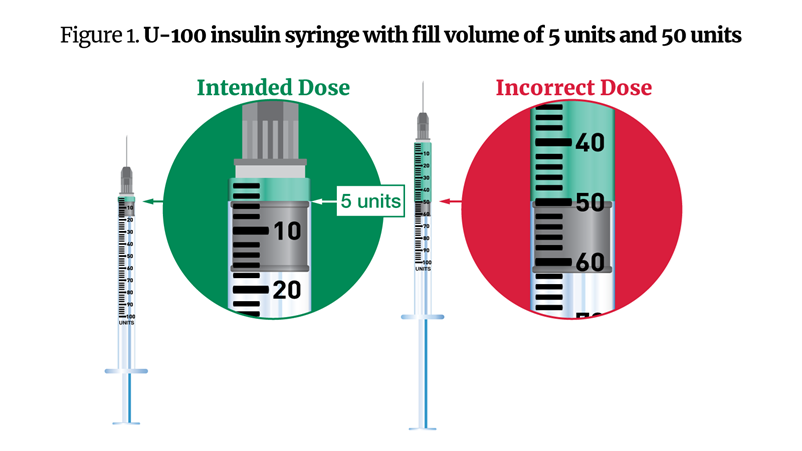
The above figure illustrates a common dosing error. Patients often confuse the intended dose of 5 units with an incorrect dose of 50 units due to the syringe's scale.
Recommendations for Patients and Providers
To mitigate these risks, the FDA recommends that patients and health care providers take several precautions:
- Clear Communication: Ensure patients understand how to measure and administer their dose correctly.
- Appropriate Tools: Provide patients with the correct syringe size for their prescribed dose.
- Vigilance in Prescribing: Health care providers should double-check their calculations and consult with compounders if there is any uncertainty about the correct dosage.
Summary
The use of compounded semaglutide presents significant risks that can lead to serious health complications. Both patients and health care providers need to be aware of these dangers and take proactive steps to ensure the safe administration of this medication. The FDA urges anyone experiencing adverse effects or dosing errors to report them to the MedWatch Adverse Event Reporting Program.
For those managing type 2 diabetes or considering semaglutide for weight loss, it is crucial to rely on FDA-approved products and seek guidance from health care professionals to avoid these potential pitfalls. By staying informed and vigilant, patients can better manage their health and avoid the serious risks associated with compounded medications.
References
- FDA Press Release: "FDA alerts health care providers, compounders and patients of dosing errors associated with compounded injectable semaglutide products."
- Reuters Article: "US FDA flags dosing risks from compounded versions of Novo's weight-loss drug."
- diaTribe Article: "FDA Warns Against Compounded Semaglutide for Diabetes, Weight Loss."
FDA MedWatch
https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program
Frequently Asked Questions
How can I tell if my semaglutide medication is compounded or FDA-approved?
Check the packaging and labeling of your medication. FDA-approved semaglutide products, such as OZEMPIC, RYBELSUS, and WEGOVY come in standardized doses and clear packaging. If in doubt, ask your healthcare provider or pharmacist.
What should I do if I suspect I have taken the wrong dose of semaglutide?
If you suspect an overdose or incorrect dose, seek medical attention immediately. Contact your healthcare provider for guidance and monitor for symptoms such as severe nausea, vomiting, or other adverse effects.
Are there specific signs or symptoms of a semaglutide overdose?
Yes, symptoms of a semaglutide overdose may include severe nausea, vomiting, abdominal pain, dehydration, fainting, headache, migraine, acute pancreatitis, and gallstones. Seek medical help if you experience any of these symptoms.
Can compounded semaglutide be safely used for weight loss?
While some patients use compounded semaglutide for weight loss, it carries higher risks compared to FDA-approved versions. Due to varying concentrations and lack of standardized dosing, compounded semaglutide should be used cautiously and only under medical supervision.
What are the alternatives to compounded semaglutide?
Alternatives include FDA-approved semaglutide products like WEGOVY for weight loss and OZEMPIC for type 2 diabetes management. These products have been tested for safety and efficacy and come with standardized dosing instructions.








