

A look at the science that explains why exercise improves glucose regulation even when insulin cannot.
Abstract
Professor Juleen R. Zierath has spent more than three decades uncovering how exercise reshapes muscle metabolism and why these mechanisms matter for people with obesity or type 2 diabetes. Her work demonstrates that insulin resistance originates in skeletal muscle and that exercise can bypass this defect through alternative pathways that enhance glucose uptake. By connecting molecular biology, circadian timing, gene regulation, and real-world physiology, she has built a foundation for more personalized approaches to metabolic health. Her research offers insight into why movement remains one of the most effective tools for improving glucose regulation and overall metabolic function.
Key Points
Read more: Professor Juleen R. Zierath: Three Decades of Showing How Exercise Changes Metabolism
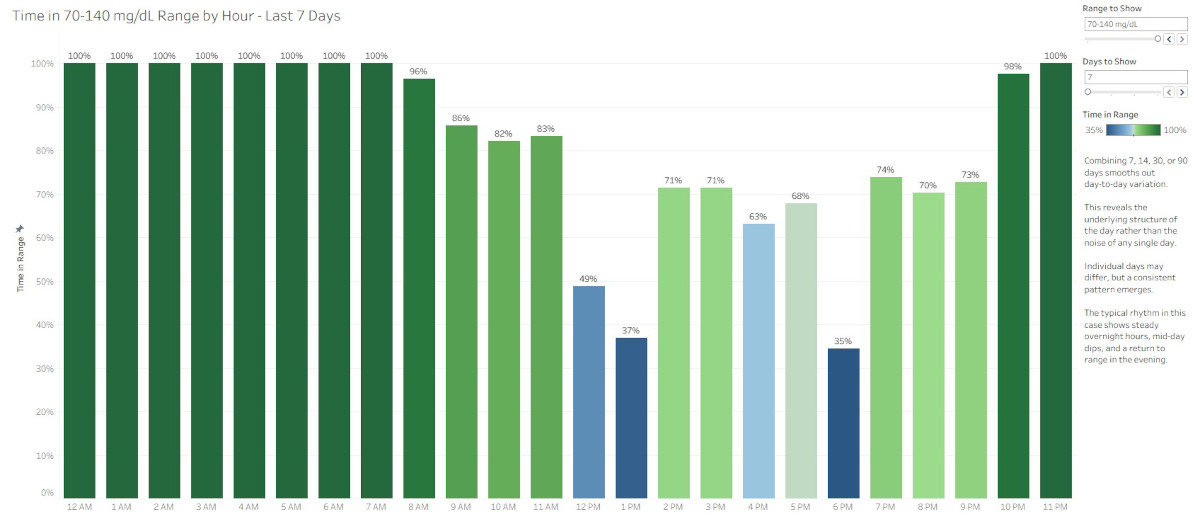
Hourly glucose visualizations replace judgment with understanding by showing when patterns occur and reducing the emotional burden of daily performance scores.
Abstract
Continuous glucose monitoring provides valuable information, but traditional daily Time in Range summaries often create unnecessary emotional pressure. This article explores how hourly Time in Range and Coefficient of Variation visualizations offer a more supportive, more insightful way to understand glucose patterns. By focusing on timing, consistency, and possibility, these visualizations shift the experience from judgment to clarity and help people interpret their data with confidence.
Key Points
A clear look at what Novo Nordisk actually said about Wegovy and why current GLP-1 therapies are not suited for OTC use.
Abstract
Discussion about Wegovy potentially moving to over-the-counter status has grown on social media, but the reporting does not support that interpretation. The statements from Novo Nordisk’s new board chair focus on strengthening consumer-oriented strategy, not pursuing OTC approval for GLP-1 therapies. This analysis reviews what was said, how it has been misinterpreted, and why current GLP-1 medications do not meet the safety, regulatory, or clinical requirements for OTC use.
Key Points
Read more: Wegovy Will Not Go OTC: What the Sources Actually Say

I completed Personalised Medicine from a Nordic Perspective through the University of Copenhagen and University of Iceland. The course explored how biobanks (collections of biological samples), health registries, and biomarkers (measurable health indicators) can be used to guide individual care, while also addressing risk communication, data protection, and broader ethical considerations.
People with diabetes often generate large amounts of data through continuous glucose monitors (CGMs), blood glucose meters (BGMs), and other wearables. This course highlights how similar kinds of data are used in healthcare systems to guide individual care, protect privacy, and support better outcomes. The material is presented in a way that makes these complex topics accessible to a broader audience, not just specialists.
This course was built and launched by two principal collaborators, Sisse Rye Ostrowski, MD, University of Copenhagen and Sædís Sævarsdóttir, MD, University of Iceland. They summed up its importance this way:
“The healthcare system is a wonderful place to be if you’re interested in data and developing algorithms. There are extremely complex data like omics data, register data, and data from wearables with all kinds of measurements you could possibly imagine. So, the healthcare field is the data playground of the future.” - Ostrowski
“We want people to understand the challenges involved and how collaboration and technological innovation is the key to shaping the future of healthcare.” - Sævarsdóttir
Explore the Course: Personalised Medicine from a Nordic Perspective
Page 1 of 23