SURMOUNT-5 results published in NEJM show greater weight loss, similar safety
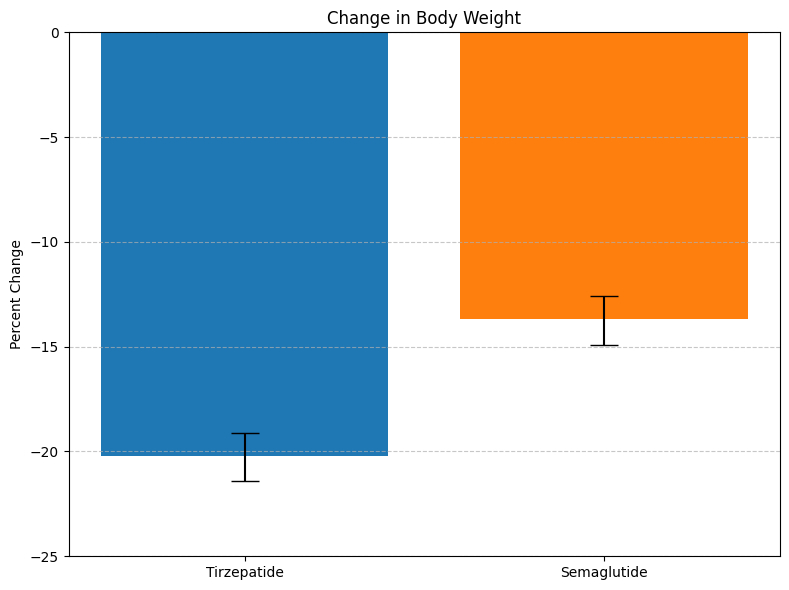
Eli Lilly’s Zepbound (tirzepatide) led to significantly greater weight loss than Novo Nordisk’s Wegovy (semaglutide) in adults with obesity and no diabetes, according to full results from the SURMOUNT-5 trial published May 11 in The New England Journal of Medicine.
Participants treated with Zepbound lost 20.2% of their body weight on average over 72 weeks, compared to 13.7% for Wegovy. Zepbound also delivered greater reductions in waist circumference (–18.4 cm vs –13.0 cm) and higher percentages of patients achieving ≥15%, ≥20%, and ≥25% weight loss.
Despite its more pronounced effect, Zepbound demonstrated a comparable safety profile to Wegovy. Gastrointestinal events were the most common side effects, with treatment discontinuation rates due to adverse events at 6.1% for Zepbound and 8.0% for Wegovy.
Experts say these findings reinforce Zepbound’s status as a leading option in obesity care. Dr. Louis Aronne, principal investigator of the study, noted that the dual GIP/GLP-1 mechanism of tirzepatide may help patients overcome plateaus seen with GLP-1 monotherapy.
As noted by Dr. Robert Gabbay in a post on LinkedIn, the trial offers new clarity not just on efficacy, but also on tolerability, side effects and adherence which are key concerns in real-world obesity management.
For more information, please visit Medscape.








