Abstract
Eli Lilly’s SYNERGY-NASH study has revealed promising results for tirzepatide in treating metabolic dysfunction-associated steatohepatitis (MASH) with stage 2 or 3 fibrosis. This development could significantly benefit individuals managing both MASH and type 2 diabetes, offering a dual approach to improve liver and metabolic health. Read on to learn more about this groundbreaking study and its potential implications for diabetes care.
Key Point
Eli Lilly’s tirzepatide shows potential as a groundbreaking treatment for MASH, significantly improving liver health and offering new hope for those with type 2 diabetes.
Eli Lilly has recently announced groundbreaking results from their SYNERGY-NASH study, revealing the potential of tirzepatide as an effective treatment for metabolic dysfunction-associated steatohepatitis (MASH) with stage 2 or 3 fibrosis. This development could mark a significant advancement in the treatment of liver diseases, especially for those also managing type 2 diabetes.
Study Highlights
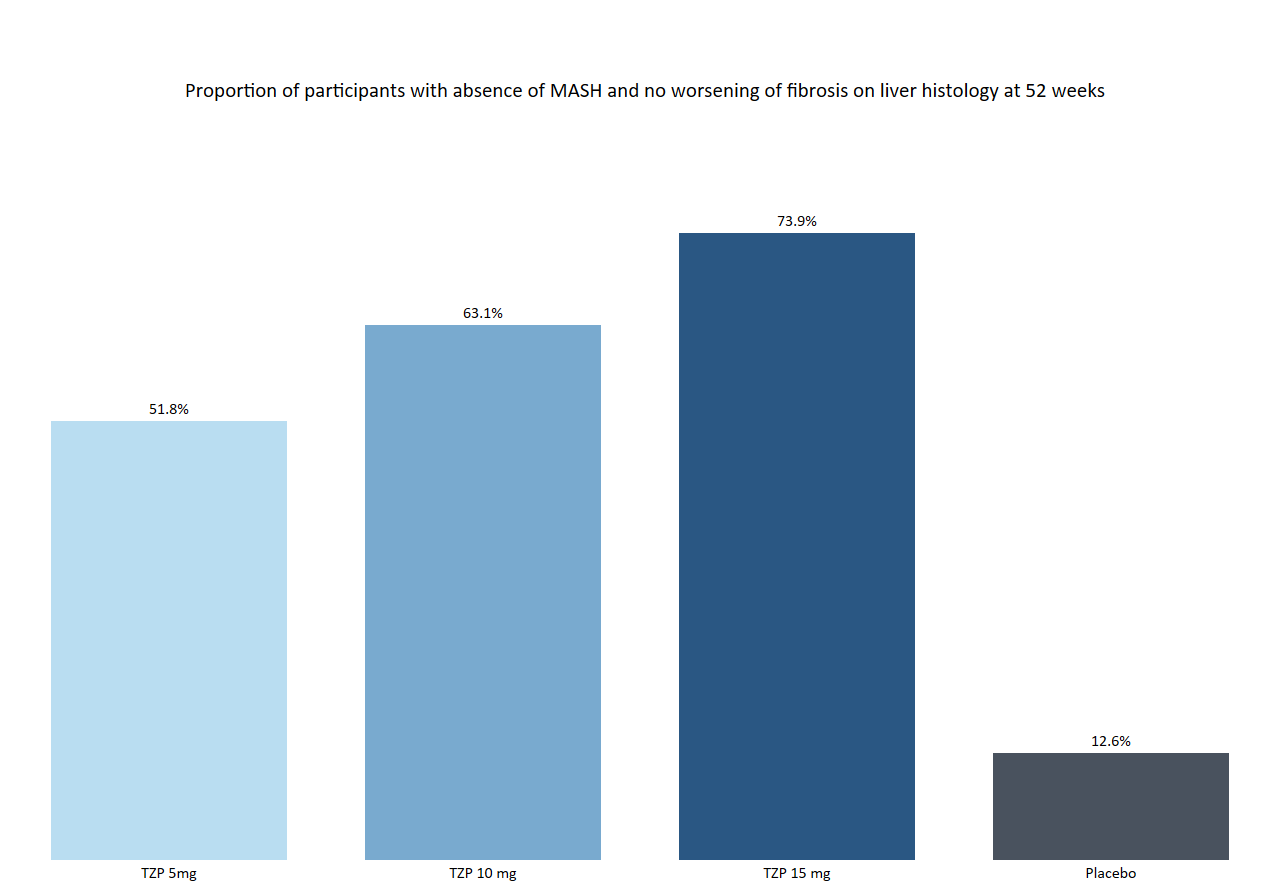
The SYNERGY-NASH study, a phase 2 clinical trial, focused on evaluating tirzepatide’s efficacy and safety in adults diagnosed with biopsy-confirmed MASH. The trial involved 190 participants, both with and without type 2 diabetes, who were administered tirzepatide in varying doses over 52 weeks.
- MASH Resolution: An impressive 51.8% of participants on a 5 mg dose, 62.8% on a 10 mg dose, and 73.3% on a 15 mg dose achieved MASH resolution without worsening fibrosis. This is in stark contrast to the 13.2% of participants in the placebo group.
- Fibrosis Improvement: More than half of the participants on tirzepatide showed significant improvement in fibrosis stages, with 59.1% (5 mg), 53.3% (10 mg), and 54.2% (15 mg) achieving a 1-stage or greater improvement without MASH worsening, compared to 32.8% in the placebo group.
- Additional Benefits: The study also highlighted improvements in body weight and various liver health markers, indicating tirzepatide's comprehensive benefits.
Safety and Tolerability
Tirzepatide's safety profile in the SYNERGY-NASH study was consistent with previous trials, with the most common side effects being mild to moderate gastrointestinal issues such as nausea, diarrhea, and decreased appetite. These findings reinforce the drug’s potential as a safe and effective treatment option.
Significance for Type 2 Diabetes Patients
For individuals managing type 2 diabetes, this development is particularly encouraging. MASH is a serious liver condition often associated with diabetes, leading to severe health complications and increasing the risk of liver transplantation. The potential approval of tirzepatide for MASH could provide a dual benefit for those battling both conditions, offering a single drug to manage liver health alongside diabetes.
Looking Ahead
Eli Lilly is actively engaging with regulatory authorities to advance tirzepatide as a treatment for MASH. If approved, this could be a game-changer, offering hope to millions affected by this debilitating liver condition.
What This Means for You
For those living with type 2 diabetes, the potential approval of tirzepatide for MASH could mean:
- Improved Liver Health: Enhanced management of liver conditions associated with diabetes.
- Comprehensive Treatment: A medication that addresses both diabetes and liver health, simplifying treatment regimens.
- Better Outcomes: Reduced risk of severe complications and improved overall health and quality of life.
This development could mark a significant advancement in the treatment of liver diseases, especially for those also managing type 2 diabetes. The positive results from the SYNERGY-NASH study demonstrate tirzepatide's potential as an effective treatment for MASH. If approved by the FDA, this could not only mark a significant advancement in liver disease therapy but also provide substantial market and revenue growth opportunities for Eli Lilly.
Further Reading
SYNERGY-NASH: Tirzepatide, a GIP/GLP-1 RA, Resolves MASH Without Worsening Fibrosis
Frequently Asked Questions
What is metabolic dysfunction-associated steatohepatitis (MASH), and how is it diagnosed?
MASH, formerly known as non-alcoholic steatohepatitis (NASH), is a severe form of fatty liver disease characterized by inflammation and damage to liver cells, along with fat accumulation in the liver. It is commonly associated with obesity, type 2 diabetes, and metabolic syndrome. Diagnosis typically involves a combination of medical history, blood tests, imaging studies (like ultrasound or MRI), and a liver biopsy to confirm the presence of inflammation and fibrosis.
How does tirzepatide work to treat MASH and type 2 diabetes simultaneously?
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. It works by activating receptors in the body that regulate blood sugar levels and appetite. This dual action helps improve insulin sensitivity, reduce blood sugar levels, decrease body weight, and potentially reduce liver fat and inflammation, thereby addressing both type 2 diabetes and MASH.
How does tirzepatide compare to other treatments currently available for MASH and type 2 diabetes?
Tirzepatide has shown promising results in improving liver health and managing blood sugar levels more effectively than some existing treatments. Its dual action on GIP and GLP-1 receptors makes it unique compared to other GLP-1 receptor agonists or diabetes medications. However, direct comparisons with other treatments require more extensive studies.
How soon can patients expect to see improvements in their liver health and diabetes management after starting tirzepatide?
In clinical studies, significant improvements in liver health and blood sugar levels were observed within 52 weeks of treatment with tirzepatide. Individual results may vary, and patients should consult their healthcare provider for personalized timelines.
Can tirzepatide be used in patients with other liver conditions or only those with MASH?
Currently, tirzepatide is being studied specifically for MASH. Its use in other liver conditions has not been established. Patients with other liver diseases should consult their healthcare provider for appropriate treatments.








