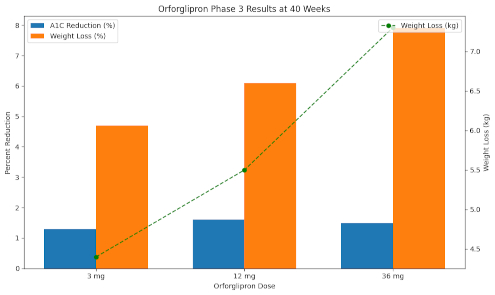
Eli Lilly’s once-daily oral GLP-1 candidate, orforglipron, has achieved statistically significant results in its first phase 3 trial, positioning the company as a front-runner in the oral GLP-1 space. The trial showed A1C reductions of up to 1.6% and weight loss of up to 16 pounds (7.9%) over 40 weeks, with a safety profile consistent with injectable GLP-1 drugs like Ozempic. Lilly plans to seek approval for obesity later this year, followed by a type 2 diabetes submission in 2026.
The readout came just days after Pfizer scrapped its competing oral GLP-1 candidate, danuglipron, due to a potential drug-induced liver injury seen in one patient. Although the case resolved after treatment was halted, Pfizer discontinued the program following regulatory input and internal review.
The timing underscores Lilly’s clinical and commercial momentum in the diabetes and obesity market. Analysts now expect Lilly’s Mounjaro, Zepbound, and upcoming candidates like orforglipron and retatrutide to continue gaining ground on Novo Nordisk’s semaglutide products, with BMO Capital Markets downgrading Novo’s stock in response to Lilly’s latest data.
Sources:
Fierce Biotech - Lilly
Fierce Biotech - Pfizer
Fierce Pharma - Lilly vs. Novo Nordisk








