
The 5K@EASD virtual 5K run/walk event is coming soon! Registration opens Monday July 15.
Registration is free.
The 5K@EASD Virtual Challenge will bring together people across the globe as well as EASD attendees to emphasize the need for increased physical activity to help prevent diabetes and diabetes complications. This disease awareness activity provides participants with the opportunity to raise public awareness about the importance of a healthy lifestyle in preventing and controlling diabetes.
The 5K@EASD is sponsored by Novo Nordisk and the World Diabetes Foundation. It takes place in conjunction with the European Association for the Study of Diabetes (EASD) annual conference.
I do the walk on the treadmill at the gym. This way I can have my earbuds in, easily track my progress and not have any interruptions.
Upload your race time any time between the race start and end dates.
5K@EASD Virtual Challenge
https://www.easd5k.com/
September 7 through September 11, 2024
Registration opens July 15. Personalized bibs will be available August 7. Finisher certificates will be available September 8.
Continue driving change in diabetes by running or walking the 5K@EASD in Madrid or from your home city!
#EASD2024 #5KEASD #DiabetesAwareness #DrivingChange

Abstract
Novo Nordisk's Awiqli (insulin icodec) is a pioneering once-weekly basal insulin designed to enhance insulin therapy convenience and adherence for people with diabetes. Despite receiving regulatory approval in Canada and the European Union, Awiqli faces hurdles in the United States due to hypoglycemia concerns, particularly for type 1 diabetes patients.
Key Points
Read more: Regulatory Hurdles for Awiqli: Approval in Canada and EU, Concerns in the US
Abstract
Eli Lilly’s tirzepatide, known as Mounjaro for type 2 diabetes and Zepbound for obesity, has shown significant efficacy in treating obstructive sleep apnea (OSA) in the SURMOUNT-OSA trial. This groundbreaking study revealed that tirzepatide not only reduces the severity of OSA but also offers substantial cardiometabolic benefits, potentially transforming the treatment landscape for this common condition.
Key Points
Read more: Tirzepatide’s Efficacy in Treating Obstructive Sleep Apnea
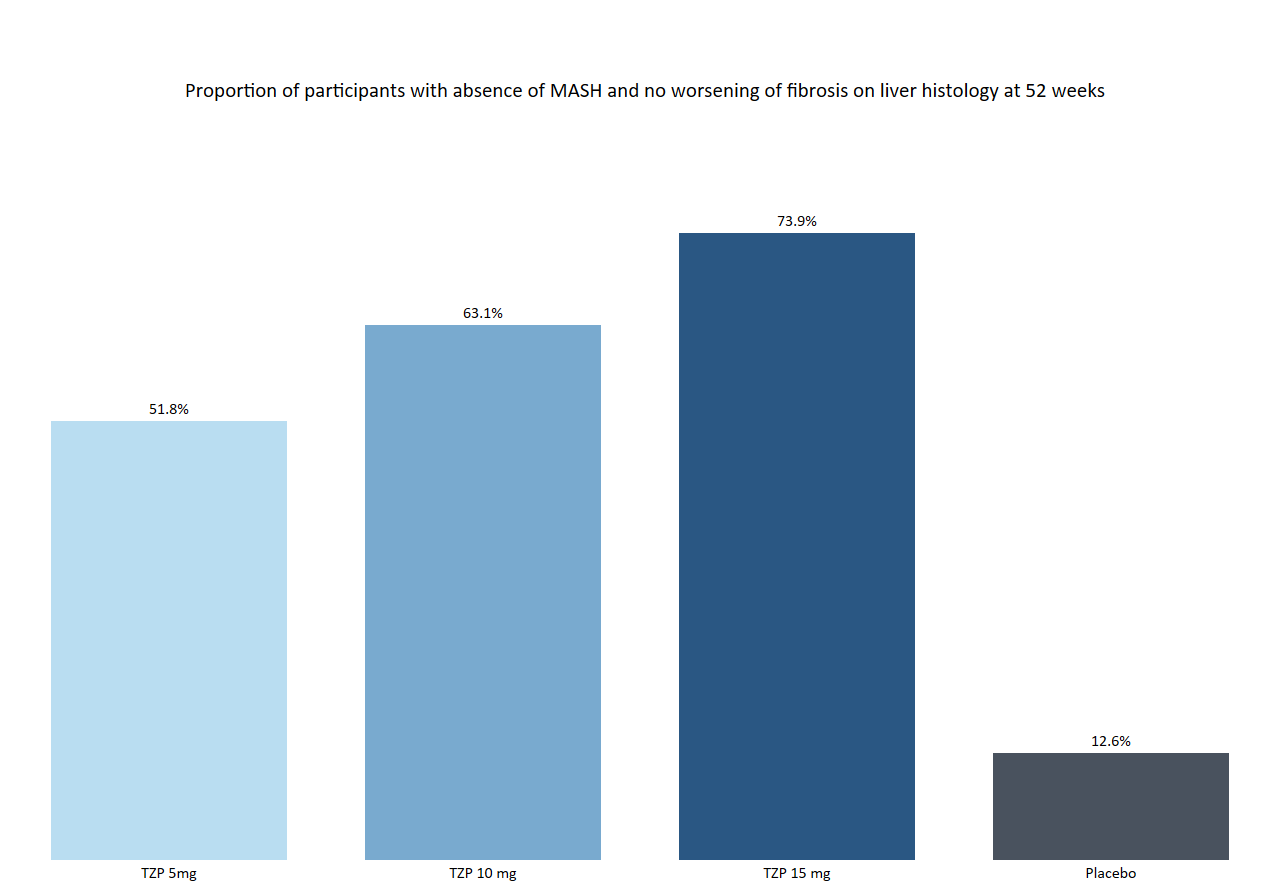
Abstract
Eli Lilly’s SYNERGY-NASH study has revealed promising results for tirzepatide in treating metabolic dysfunction-associated steatohepatitis (MASH) with stage 2 or 3 fibrosis. This development could significantly benefit individuals managing both MASH and type 2 diabetes, offering a dual approach to improve liver and metabolic health. Read on to learn more about this groundbreaking study and its potential implications for diabetes care.
Key Point
Eli Lilly’s tirzepatide shows potential as a groundbreaking treatment for MASH, significantly improving liver health and offering new hope for those with type 2 diabetes.
Read more: Eli Lilly’s Tirzepatide Shows Promising Results in MASH Treatment
Page 5 of 23