
Abstract
Viewing type 2 diabetes as a cardiovascular disease provides a better understanding of the condition and underscores the importance of integrated care. By focusing on both metabolic and cardiovascular health, we can better manage diabetes, reduce complications, and improve the overall quality of life for patients. It’s a beneficial and effective holistic approach to managing diabetes.
Managing diabetes isn't just about controlling blood sugar levels; it's about taking care of your entire cardiovascular system. Incorporating both medication and lifestyle changes significantly reduces the risk of heart attacks, strokes, and kidney disease which helps us to live longer, healthier lives.
Key Points
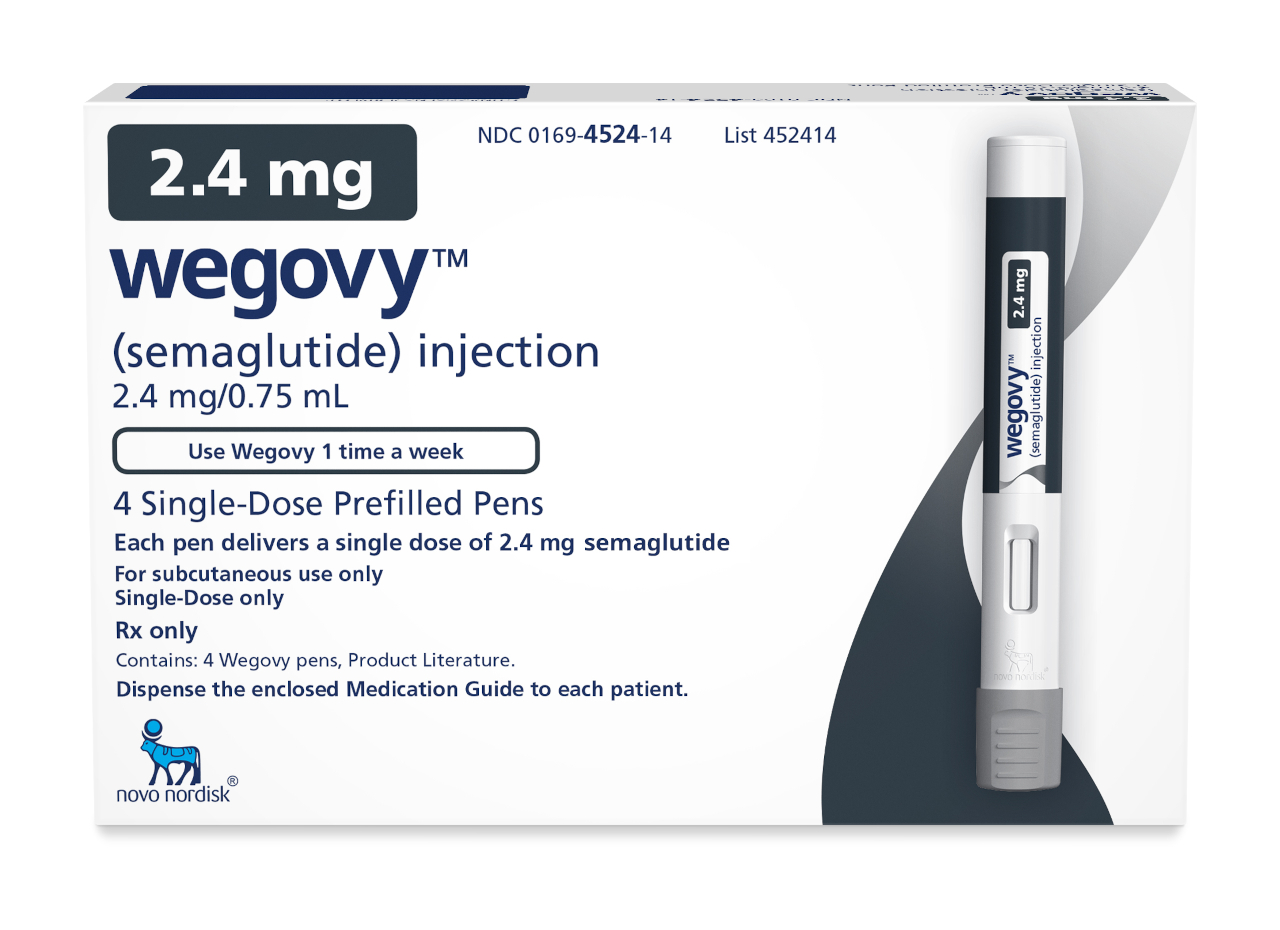
In recent years, the fight against obesity has seen significant advancements, with Wegovy (semaglutide) emerging as a leading player. Recent studies have demonstrated not only its effectiveness in sustained weight loss but also its potential in reducing cardiovascular risk.
Long-Term Weight Loss with Wegovy
1. Demonstrated Sustained Weight Loss
Wegovy has been proven to help patients achieve and maintain significant weight loss over an extended period. In the SELECT trial, participants maintained an average weight loss of 10% after four years of continuous use. This sustained weight loss is a breakthrough, given that weight loss typically plateaus after about 65 weeks but remains stable with continued treatment.
2. Impressive Trial Results
The SELECT trial revealed that 68% of participants lost at least 5% of their body weight, while 23% achieved a reduction of at least 15%, results that demonstrate significant and enduring weight management outcomes.
3. Comparative Data
Earlier trials indicated an even higher average weight loss of approximately 15% over 68 weeks. Although the SELECT trial, focusing on cardiovascular outcomes, showed a slightly lower average weight loss, this difference is attributed to variations in trial design and participant selection.
4. Safety and Tolerability
Wegovy's safety profile is solid, with common side effects including gastrointestinal issues such as nausea and diarrhea. Despite some patients discontinuing due to these side effects, serious adverse events were lower in the semaglutide group compared to placebo, primarily due to fewer major cardiovascular events.
FDA Approval for Cardiovascular Risk Reduction
1. Landmark FDA Approval
The FDA's recent approval of Wegovy to reduce the risk of major adverse cardiovascular events (MACE) is significant. This approval is based on the SELECT trial, which demonstrated a 20% reduction in the risk of MACE, including heart attack, stroke, and cardiovascular death, compared to placebo.
2. Cardiovascular Benefits Beyond Weight Loss
Cardiovascular benefits were observed even in participants who did not achieve significant weight loss, suggesting additional mechanisms of action, such as reduced inflammation and improved blood glucose control.
3. Implications for Treatment
This approval represents a significant advancement in treating patients with obesity and cardiovascular disease, providing a new, effective option to reduce cardiovascular risk. The new indication is expected to lead to broader insurance coverage and increased accessibility for patients, although cost and supply challenges persist.
Addressing the Cost Barrier
1. The High Cost of Wegovy
Despite its benefits, the high cost of Wegovy remains a significant barrier to widespread adoption. In the United States, Wegovy's list price is approximately $15,600 per year, which can be a substantial financial burden for many patients. Even with insurance discounts, the cost can be prohibitive.
2. Impact on Healthcare Systems
The financial impact of these drugs on health insurers and governments is substantial. High prices could limit access to those who most need the treatment, potentially leading to inequalities in healthcare. The argument for making these drugs more affordable is strong, especially given the broader health benefits they offer, including reduced cardiovascular risks and potential improvements in conditions like kidney disease and dementia.
3. Calls for Price Reductions
There have been increasing calls for pharmaceutical companies to reduce the prices of these essential medications. Senator Bernie Sanders has highlighted the need for price reductions, suggesting that the cost in the U.S. should be aligned more closely with lower prices seen in other countries like Denmark.
4. Outlook
As the benefits of Wegovy become more apparent, the friction between its effectiveness and affordability will likely push for a more sustainable pricing model. Innovative pricing strategies and cost-spreading deals are being discussed to make these life-changing drugs accessible to a broader population.
Summary
Wegovy's dual benefits of sustained weight loss and significant cardiovascular risk reduction make it a valuable treatment option for patients with obesity and cardiovascular disease. The recent FDA approval marks a milestone in obesity management, promising to enhance the quality of life for many patients while addressing critical health risks associated with obesity. However, for Wegovy to reach its full potential and benefit those who most need it, the cost barrier must be addressed. Lowering the price and improving accessibility will ensure that more individuals benefit from treatment, which will contribute to better public health outcomes.
Frequently Asked Questions
What is the recommended dose of Wegovy for the most significant weight loss effect?
The most significant weight loss effect was observed with a 2.4 mg dose of Wegovy, which is why it received FDA approval, unlike Ozempic, which has a maximum dose of 2 mg.
How does Wegovy compare to other weight loss medications on the market?
Wegovy has shown superior long-term weight loss and cardiovascular benefits compared to many other weight loss medications, making it a leading option in obesity management.
Can Wegovy be used by individuals without cardiovascular disease?
Yes, Wegovy can be used by individuals without cardiovascular disease and is primarily indicated for weight management in adults with obesity or overweight conditions.
What lifestyle changes should be made while taking Wegovy?
Patients taking Wegovy are advised to adopt a healthy diet and increase physical activity to maximize weight loss and overall health benefits.
How is Wegovy administered?
Wegovy is a once-weekly subcutaneous injection by way of a single-use pre-filled disposable pen. Patients should follow their healthcare provider's instructions on dosage and injection technique.

Eli Lilly has announced positive phase 3 results for its once-weekly insulin, efsitora alfa (efsitora). This news represents a significant advancement in diabetes management, potentially transforming treatment for millions of people living with type 2 diabetes.
Phase 3 Clinical Trials: QWINT-2 and QWINT-4
Eli Lilly’s phase 3 trials, QWINT-2 and QWINT-4 evaluated the efficacy and safety of efsitora. The QWINT-2 trial compared efsitora to Novo Nordisk’s daily insulin degludec (Tresiba) over 52 weeks in insulin-naïve adults. The trial successfully met its primary endpoint, demonstrating that efsitora was non-inferior (at least as good as) to degludec in reducing HbA1c levels. Efsitora reduced HbA1c by 1.34%, while degludec achieved a 1.26% reduction, resulting in final HbA1c levels of 6.87% and 6.95%, respectively.
The QWINT-4 trial compared efsitora to insulin glargine (Lantus) over 26 weeks in adults previously treated with basal insulin and mealtime insulin. Both treatments reduced HbA1c by 1.07%, demonstrating comparable efficacy. Importantly, efsitora was well-tolerated in both trials, though it did show slightly higher rates of hypoglycemic events compared to the daily insulins. Hypoglycemia is always a possibility for anyone taking insulin.
The Competitive Landscape: Novo Nordisk's Insulin Icodec
Eli Lilly's success with efsitora comes amid fierce competition with Novo Nordisk, another principal producer of insulin. Novo Nordisk's once-weekly insulin, Icodec (Awiqli®), is currently under FDA review, with approval anticipated later this year. Icodec has already demonstrated promising results, achieving superior blood sugar reduction and increased Time in Range compared to daily basal insulins in clinical trials.
Comparing Efsitora Alfa and Icodec
Both efsitora and Icodec aim to reduce the burden of daily injections for people with diabetes, offering a once-weekly alternative. Key comparisons include:
The Future of Diabetes Treatment
Once-weekly insulins like efsitora and Icodec mark a noteworthy advancement in diabetes treatment. These innovations will significantly reduce the daily burden of managing diabetes, potentially improving treatment adherence and health outcomes. As both Eli Lilly and Novo Nordisk continue to innovate in diabetes care, patients can look forward to more convenient and effective treatment options.
With regulatory approvals coming, the future of diabetes management looks brighter than ever.
Frequently Asked Questions
How soon can patients expect to see improvements in their blood sugar levels after starting efsitora alfa?
Patients may begin to see improvements in blood sugar levels within the first few weeks of starting efsitora alfa, as it gradually stabilizes glucose levels over time.
Are there any dietary or lifestyle changes recommended when switching to once-weekly insulin?
No specific dietary or lifestyle changes are required when switching to once-weekly insulin, but maintaining a healthy diet and regular exercise routine is always beneficial for diabetes management.
How do once-weekly insulins affect insulin sensitivity compared to daily insulins?
Once-weekly insulins like efsitora alfa are designed to provide stable insulin levels throughout the week, which may help maintain insulin sensitivity similarly to daily insulins.
How do patients and caregivers feel about switching to once-weekly insulin?
Feedback from patients and caregivers has been positive, highlighting the convenience and reduced injection burden as significant benefits of once-weekly insulin.
How will once-weekly insulins be integrated into existing diabetes treatment guidelines?
Once-weekly insulins are expected to be incorporated into diabetes treatment guidelines as an alternative to daily basal insulins, especially for patients who struggle with adherence to daily injections.

In a landmark move, New York has become a beacon of hope for millions of Americans, particularly those grappling with the daily challenges of diabetes. The state has officially eliminated cost-sharing for insulin in state-regulated health plans, a decision that is being hailed as a transformative step towards medication affordability and accessibility.
A Victory for Diabetes Advocates
The American Diabetes Association (ADA), alongside a dedicated community of Diabetes Advocates, has been at the forefront of this battle, striving to make diabetes treatment more affordable. Their efforts have borne fruit in 25 states and the District of Columbia, but New York's recent action is particularly noteworthy. It represents a significant victory in the fight against the diabetes epidemic, a fight that the ADA has been leading for over eight decades.
The Impact of High Medical Costs
For individuals living with diabetes, the cost of insulin can be a heavy financial burden. Over the past ten years, medical costs for Americans with diabetes have surged by 35%. These individuals face medical expenses that are, on average, 2.6 times higher than those without diabetes. The financial strain is so severe that one in six Americans have resorted to rationing their insulin, taking less than needed or skipping doses, which can lead to life-threatening complications.
New York's Bold Initiative
New York's decision to eliminate insulin copays is a critical step towards greater health equity. This policy change is expected to support a healthier future for the over 1.8 million adult New Yorkers with diagnosed diabetes.
The Numbers Speak Volumes
In New York alone, approximately 11.7% of the adult population, which translates to roughly 1.8 million adults, have been diagnosed with diabetes. Each year, an estimated 92,000 New Yorkers receive a new diabetes diagnosis. The elimination of insulin copays is a significant financial and emotional relief for these individuals and their families.
Summary
New York's action to eliminate insulin copays marks a significant milestone in the journey towards making life-saving medication accessible and affordable. It's a testament to what can be achieved through persistent advocacy and compassionate governance. As we celebrate this victory, let's also remember that the fight continues, and together, we can make a difference in the lives of those affected by diabetes.
American Diabetes Association Press Release
Frequently Asked Questions
How will this policy be funded?
The policy will be funded through state healthcare budgets and possibly federal contributions or subsidies.
What types of insulin products are covered under this policy?
It covers all basic types of insulin required for diabetes management, including rapid-acting and long-acting insulins.
Does this policy apply to all health insurance plans in New York?
The policy applies only to state-regulated health plans, not federal or private plans not under state regulation.
What are the expected impacts of this policy on insulin prices nationally?
The policy might set a precedent for other states but its direct impact on national insulin prices is unclear.
How will out-of-pocket costs for diabetics change overall due to this policy?
Out-of-pocket costs for insulin under covered plans will be significantly reduced or eliminated, improving affordability.
Page 6 of 23